Unauthorized products may pose serious health risks (Part 1)Health ProductsFeb 18th, 2021 @ 12:00 AM
Basic Details
Summary
Issue
Products may contain dangerous ingredients such as prescription drugs that are not listed on the label, or the label may indicate a dangerous ingredient or combination of ingredients.
Product
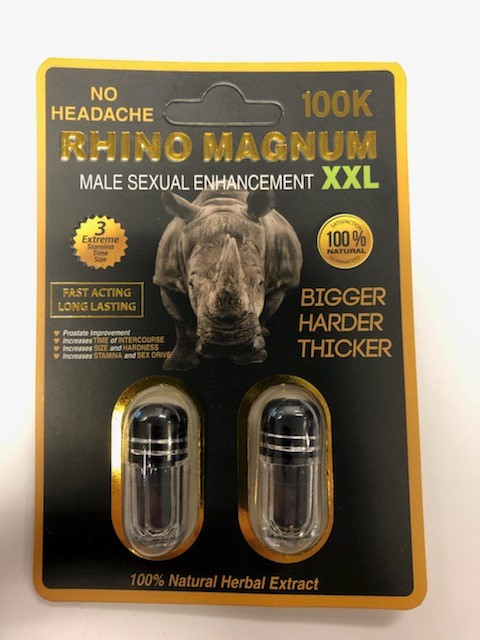
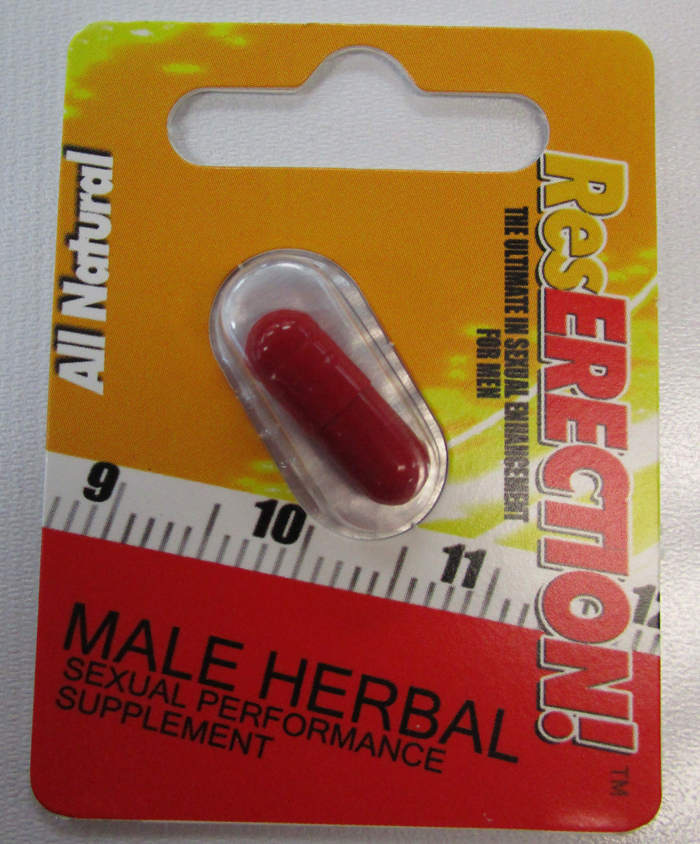
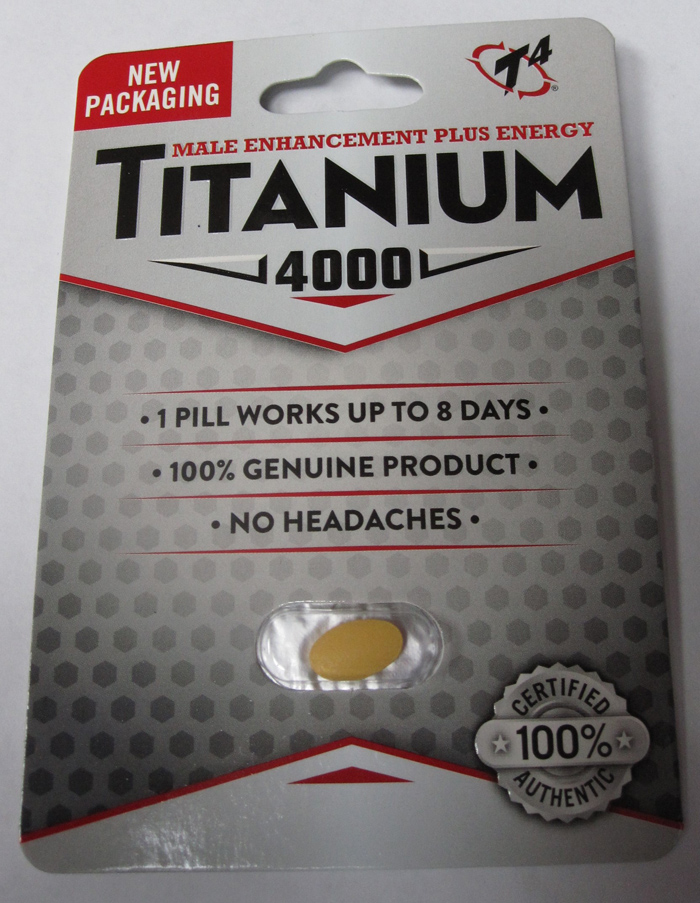
Various unauthorized health products promoted for sexual enhancement, weight loss, as a workout aid, as “poppers,” or for lightening skin or treating skin conditions (such as eczema or psoriasis).
What to do
Stop using these products and consult your healthcare professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
ImagesClick to enlarge
Products
| Product description | Common name |
|---|---|
Issue
Health Canada is advising Canadians about unauthorized health products that may pose serious health risks. The table below is updated when Health Canada finds unauthorized health products that are promoted for sexual enhancement, weight loss, as a workout aid, as “poppers,” or for lightening skin or treating skin conditions (such as eczema or psoriasis). These products are labelled to contain or have been tested and found to contain dangerous ingredients. Links to previous tables with affected products are also available below. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
Health Canada maintains this page so that Canadians can easily identify products they may have purchased and take appropriate action. Canadians are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database. What companies should know:
What you should do
BackgroundDapoxetine is a drug used to treat premature ejaculation and is not authorized for sale in Canada. Side effects include fainting or loss of consciousness, dizziness, changes in blood pressure, blurred vision, seizures, headache, diarrhea and nausea. In particular, dapoxetine should not be used by individuals who have cardiac conditions, liver disease, a history of mania or bipolar disorder, or who are taking certain other medications, including monoamine oxidase inhibitors, antidepressants, and a number of other drugs and herbal products that can interact with dapoxetine. Flibanserin is a prescription drug used to treat hypoactive (low) sexual desire disorder (HSDD) in premenopausal women who have not had problems with low sexual desire in the past and should only be used under the supervision of a health care professional. Women should not take flibanserin if they have liver problems, are pregnant or breastfeeding, if they are taking certain other medicines, or if they have low blood pressure and drink alcohol. Common side effects include dizziness, sleepiness, nausea and tiredness. Serious side effects include fainting, low blood pressure, abnormally fast heartbeat, and mental health changes such as dissociation from reality, hallucination, intensely excited mood and racing thoughts. The risk of side effects increases with the amount taken (dose). "Poppers" is a slang term for products that contain alkyl nitrites. Despite being labelled for various uses such as leather cleaners, room odourizers or liquid incense, these products are inhaled or ingested by consumers for recreational purposes. Alkyl nitrites, such as amyl nitrite, butyl nitrite and isobutyl nitrite are prescription drugs and should be used only under the supervision of a healthcare professional. Products containing alkyl nitrites may pose serious risks, including death, depending on the amount used, how frequently they are used and how long they are used for, as well as the person's health and the other medications they may be taking. Since it is difficult to control how much is inhaled, people can accidentally overdose. Swallowing these products can lead to serious medical complications and may be fatal. People with certain medical conditions (including recent head trauma, bleeding into the head, glaucoma, or heart disease) and those taking certain medications (particularly drugs used to treat erectile dysfunction, and other drugs such as high blood pressure medications, certain migraine drugs, and high doses of aspirin) or illicit drugs are at particular risk. Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g. nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Desmethyl carbodenafil and dithiodesmethyl carbodenafil, and Hydroxythiohomosildenafil are unauthorized substances that are similar to sildenafil and may pose similar health risks. Tadalafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) since it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Aminotadalafil is an unauthorized substance that is similar to tadalafil and may pose similar health risks. Yohimbine is a prescription drug and should be used only under the supervision of a health care professional. Yohimbine is derived from yohimbe, a bark extract. The use of yohimbine or yohimbe may result in serious adverse reactions particularly in people with high blood pressure, or heart, kidney or liver disease. Side effects include increased blood pressure and heart rate, anxiety, dizziness, tremors, headache, nausea and sleep disorders. It should not be used by children, or pregnant or nursing women. Related alertsUnauthorized products may pose serious health risks (November 17, 2017 to May 23, 2018) 2017-11-17 | Natural health products Type of communication: Advisory Unauthorized products may pose serious health risks (June 5, 2018 to November 7, 2018) 2018-06-05 | Natural health products Type of communication: Advisory Unauthorized products may pose serious health risks (December 10, 2018 to April 17, 2019) 2018-12-10 | Natural health products Type of communication: Advisory Unauthorized products may pose serious health risks (May 17, 2019 - Part 1) 2019-05-17 | Natural health products Type of communication: Advisory Unauthorized products may pose serious health risks (May 17, 2019 - Part 2) 2019-05-17 | Natural health products Type of communication: Advisory Unauthorized products may pose serious health risks (June 11, 2019 to July 26, 2019) 2019-06-11 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (August 12, 2019 to September 18, 2019) 2019-08-12 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (October 2, 2019 to November 19, 2019) 2019-10-02 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (December 9, 2019 to January, 17 2020) 2019-12-09 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (February 10, 2020 to March 13, 2020) 2020-02-10 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (September 16, 2020: Part 1) 2020-09-16 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (September 16, 2020: Part 2) 2020-09-16 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (October 21, 2020) 2020-10-21 | Drugs Type of communication: Advisory Unauthorized products may pose serious health risks (November 25, 2020) 2020-11-25 | Drugs Type of communication: Advisory For more informationMedia enquiriesPublic enquiries
|